INTRODUCTION
The growing resistance to antibiotics has led to the design and evaluation of new alternatives against various pathogens of clinical interest, including Staphylococcus aureus and Klebsiella pneumoniae.
In 2021, the World Health Organization (WHO) highlighted that antimicrobial resistance represents one of the 10 main threats to public health, emphasizing the increase in deaths among children and young adults(1). Infectious diseases have been one of the leading causes of morbidity and mortality in underdeveloped countries, so adequate and timely treatment will have a favorable impact on health indicators2. In this sense, one of the main microorganisms that cause a wide variety of infections is Staphylococcus aureus, which is an important pathogen. Human bacterial that cause a wide variety of clinical manifestations (pneumonia, endocarditis infectious, sepsis, infection in soft tissues, among others), which can be acquired in common environments (growing) and hospitals (more common) (3. For its part, Klebsiella pneumoniae is a Gramnegative bacterial pathogen that is usually associated with nosocomial infections, urinary tract infections, respiratory tract infections, and bloodstream infections, mainly affecting immunosuppressed people4),(5. Motivated so to the endurance antimicrobial, disciplines such as Bionanotechnology have emerged, which have developed and evaluated a diversity of nanomaterials with antimicrobial capacity. Among these nanomaterials, metallic nanoparticles (gold, copper, silver, iron, cadmium) stand out, which have been shown to cause significant damage to the cell wall, in addition to the alteration of its vital cellular processes, resulting in the inhibition of microbial growth producing a biocidal effect6),(7.
The various technologies applied for the synthesis of metal nanoparticles are based on physical and chemical processes, which present disadvantages not only from the economic point of view, (high costs), either technological (need for specialized equipment), but also environmental damage due to the requirement of using highly toxic organic solvents and other highly aggressive chemical compounds8. Therefore, currently technological efforts are based in methods biological, which are more feasible and friendly to the environment, where the use of plants, isolated biomolecules such as flavonoids, plant extracts, cells and tissues is required, for the synthesis of NPs9),(10. In accordance with the above and taking into account the increase in drug resistance by bacterial agents, it is advisable to synthesize new nanomaterials that can be implemented as treatments with antibacterial effects, to treat diseases effectively and safely. For this reason, the present work aimed to evaluate the Effect of the Biosynthesis of Silver Nanoparticles (AgNPs) and Lithium (LiNPs), obtained by reduction with quercetin on the proliferation of a strain of Staphylococcus aureus and Klebsiella pneumoniae.
EXPERIMENTAL
Biological sample
To evaluate the antibacterial effect of the NPs , two certified strains were used, one of Staphylococcus aureus ATCC 25923 provided by the Pharmacological Biochemistry laboratory of the “Dr. Francisco Triana Alonso” (BIOMED-UC) and another Klebsiella pneumoniae ATCC 700603, donated by the “Fitoquimica20” Biotechnology Laboratory.
Optimization of the synthesis of silver (AgNPs) and lithium (LiNPs) nanoparticles
The method used is based on the capacity of flavonoid-type biomolecules (biological route or green synthesis) that causes a reduction of the metal until the formation of the NPs and their stabilization by the same reducing agent. To obtain the best synthesis performance of the AgNPs, three concentrations of 0.4 silver nitrate were used; 0.8; 1.2 mM, quercetin/AgNO 3 volume ratio of 1:1, 1:2 and 2:1. For the synthesis of the LiNPs, the lithium sulfate concentrations were 0.2; 0.6 and 1 mM , respectively, with a volume ratio of quercetin (QCT)/ Li 2 SO 4 of 1:1, 1:2 and 2:1. In both cases, the pH was kept constant at 5, stabilized with 0.1 M sodium hydroxide, and the reaction temperature at 50 °C. Finally, a spectral scan of each synthesis obtained was performed, in a wavelength range from 250 to 750 nm using the Ultraviolet/Visible (UV/Vis) molecular absorption spectrophotometer, GENESIS 2011.
Determination of the size and morphology of copper nanoparticles by scanning electron microscopy (SEM)
The morphology and size of the synthesized AgNPs and LiNPs were analyzed using the JEOL/EO JSM-5600 SEM equipment at 200 μ m, 10 μ m and 2 μ m. The NPs were placed in thin films on a carbon-coated copper grid by dropping 20-50 μL of sample on the grid and then observed under the analyzer. The foreign material was cleaned with tissue paper and then allowed to dry under a mercury lamp for 4 to 5 minutes12.
Evaluation of the antibacterial activity of AgNPs and LiNPs, on Staphylococcus aureus and Klebsiella pneumonia
To start growth studies bacterial strain of S. aureus and K. pneumoniae , we proceeded to his crop in conditions aerobics to 37 ºC with constant stirring (250 rpm), for 24 hours (pre-inoculum) in 30 mL of Luria-Bertani (LB) liquid medium; This is a nutrient-rich medium consisting of: 1% tryptone, 0.5% yeast extract and 1% NaCl (pH was adjusted to 7 with NaOH). Subsequently, from this pre-inoculum, the amount necessary to inoculate 0.05 U/mL of optical density at 600 nm (OD 600 ) in the culture used for the growth curve was determined ( Elisa Microplate Reader Model W201, EMPSUN brand) (13. In this way, each growth curve test was started with the same number of bacteria in the culture medium. LB. The effect of NPs on bacterial growth was determined in the absence (control) and in the presence of different concentrations (0.2-0.4-0.6-0.8 and 1 mg/mL, respectively). In a 96-well U-bottom plate, 2µL of the solutions corresponding to the NPs and 198 µL of bacterial culture were placed. A growth control was used in all trials. Later, the growth bacterial HE determined through the D 600 for each concentration of the different concentrations every 60 minutes for up to 6 hours. Finally, the readings of DO 600 depending on the concentration of the different concentrations used. To determine the minimum inhibitory concentration (MIC), we proceeded to read the DO the 24 h of incubation, The MIC being that where the OD was equal to less than 0.2 units.
RESULTS AND DISCUSSION
Optimization of the synthesis of silver (AgNPs) and lithium (LiNPs) nanoparticles
The optimal synthesis conditions for AgNPs and LiNPs are observed in Table 1. This optimization process represents a fundamental step to obtain the highest performance of synthesis and also ensure that the NPs are stabilized by the agent itself that causes their reduction until the formation of the NPs. In this sense, AgNPs and LiNPs are stabilized by QCT through non-covalent interactions such as Van der waals forces forming besides colloids which are defined as intermediate systems between true solutions and suspensions, made up of nanoparticles of a certain material (dispersed or dispersoid phase) that are suspended in a medium of a different nature than the particle in suspension (dispersion medium) (14),(15.
Table 1 Optimization of the synthesis of nanoparticles silver (AgNPs) and lithium (LiNPs)
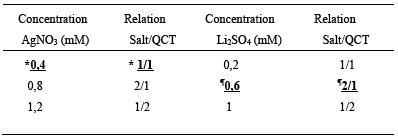
*Volume of silver nitrate salt (AgNO 3 ) 500 µL and 500 µL quercetin (QCT), ¶ 500 µL lithium sulfate (Li 2 SO 4 ) and 1000 µL of QCT
On the other hand, the AgNPs presented an absorption maximum at 420 nm, a result very similar to those reported in other studies, which indicate that the AgNPs can cause absorption peaks that vary in a range of 418 and 426 nm. About the LiNPs, there is a point to highlight, since this represents the first synthesis of LiNPs in Venezuela and one of the few in Latin America by biological route, that is, through the use of biomolecules with reducing capacity isolated from plant material, presenting a maximum absorption at 480nm, consistent with what was reported by researchers from the Physics Research Center of the University of Sonora, who obtained absorption peaks of 380, 460 and 480 nm depending on the size of the LiNPs16.
Determination of the size and morphology of nanoparticles by scanning electron microscopy (SEM)
Although surface scanning or spectral scanning provides valuable information related to the formation of NPs, it is important to know their exact size and morphology, since these two variables play a fundamental role in the antimicrobial effect, the microscopy technique Scanning electron beam (SEM) showed that the AgNPs had a size of 38 nm, with a spherical morphology (Figure 1A). According to what was indicated by Khodadadi, et al. (17, AgNPs could even present sizes less than 20 nm, considering the variability of biomolecules that could be combined to originate them. Regarding the LiNPs, the size obtained was 36 nm with a spherical shape (Figure 1B). To date there are no published articles that indicate the approximate size of LiNPs obtained by this technique (SEM), for this reason it is of great importance to use other techniques such as X-ray diffraction for the characterization of this particular type of NPs.
Assessment of the activity antibacterial of AgNPs and LiNPs, on Staphylococcus aureus and Klebsiella pneumoniae
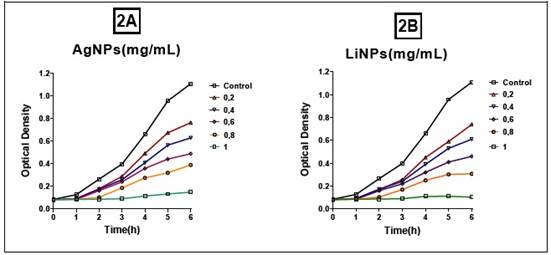
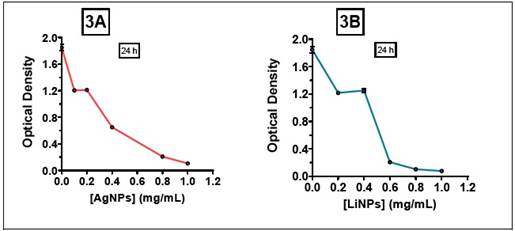
Regarding the effect of AgNPs and LiNPs on S. aureus, an inhibition of proliferation was observed for all the range of contractions employed (Figure 2A and 2B), with a minimum inhibitory concentration (MIC) close to 0.8 mg/mL for the AgNPs and a C MI close to 0.6 mg/mL for the LiNPs (Figure 3A and 3B).
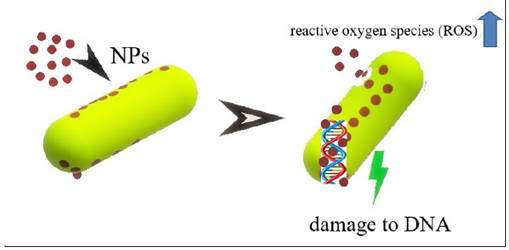
This difference may be associated with the size of the 36 nm LiNPs, which allows them to have a larger contact surface, thus increasing the antimicrobial effect through various mechanisms such as the production of reactive oxygen species (ROS), with damage to DNA and the inactivation of the bacteria's own enzymatic systems. In this sense, controlling the size of NPs is of vital importance in massifying their antimicrobial effect18. Figure 4 prepared by the authors describe the aforementioned molecular events.
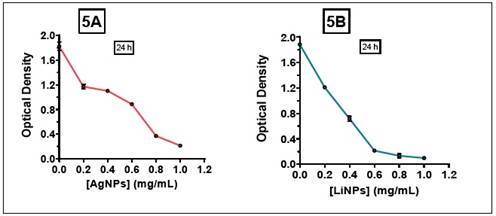
Now, comparing the effects observed with other types of NPs, the study carried out by Figueroa Calderón18),(19 using iron nanoparticles, found that FeNPs inhibited the proliferation of S. aureus with an MIC close to 0.9 mg/mL, associating this effect with the synthesis conditions. In relation to the effect against K. pneumoniae, with both NPs a positive behavior of inhibiting proliferation was observed, however, the MIC of the AgNPs was close to 1 mg/mL, unlike the LiNPs whose MIC remained close to 0 .6 mg/mL (Figure 5A and 5B).
The explanation for this behavior is not only linked to the size of AgNPs, but also because K. pneumoniae is an encapsulated microorganism. For this reason, this capsule or surface structure formed by complex exopolysaccharides provides protection to the cell from potential antimicrobials20.
CONCLUSIONS
Lithium and silver nanoparticles presented an inhibitory effect in both strains. It was evident that the behavior of NPs is linked not only to size and morphology, but also to the type of microorganism (Gramnegative or Grampositive). New alternatives such as the use of metallic nanoparticles constitute an important step in the development of new and more effective antimicrobials.












 uBio
uBio