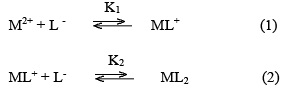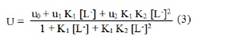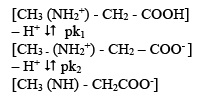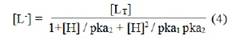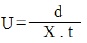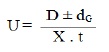INTRODUCTION
FOR a mononuclear binary complex, if a central atom (central group) M (the ‘metal’ and a ligand L have been defined, then in the following expressions K n is the stepwise formation constant, and ß n is the cumulative formation constant for the complex MLn. They can both be referred to as stability constants (stepwise and cumulative)1
K n = K (ML n-1 + L MLn)
ß n = K (M+ nLMLn)
Metal complexes play an important role in various biological systems; hence, the formation, stability and reactivity of these complexes have been an active field of research2),(3. Cadmium has been recognized as one of the most toxic environmental and industrial pollutants. It has a very long biological half-life (about thirty years in mammals), and slow excretion of Cd2+ contributes to Cd2+ accumulation in living cells, causing damages in liver, kidney, lungs and nervous system4. The accumulation of redox-active iron in mitochondria leads to oxidative damage and contributes to various neurogenerative disease, such as Friedreich’s ataxia and Parkinson’s disease5. Zinc always occurring as a divalent action in biological systems, is the second most abundant transition metal following iron, large amount of zinc (II) are likely to concentrate in nerve tissues (0.1 - 0.5 mM of brain tissue). The best role of zinc is as a structural cofactor in metalloprotein6. Cadmium, iron and zinc ions are well known for their biomedical application and toxicity7),(15. Sarcosine is an N-methyl derivative of glycine. It is a natural amino acid found in muscles and other body tissues. It is normally not detected in human blood or urine. Sarcosine has several biomedical applications16),(20.
Kiso21 has done comprehensive study on paper electrophoretic migration of metal complexes. The electrophoretic technique usually suffers from a number of defects, namely, temperature during electrophoresis, capillary flow on paper, electro-osmosis and adsorption, all effect the mobility of charged moieties22. The present technique is almost free from these negative factors and is very convenient to use. It gives results in fair agreement with the accepted literature values.
Communications23),(25 from our laboratory described new method for study of binary complexes. A search of literature indicated very few reports on complexation of cadmium (II), iron (II) and zinc (II) with sarcosine. In view of this attempt was made to establish the optimum conditions for metal(II) - sarcosine complexes formation. In, addition present paper describes a paper ionophoretic method for the determination of stability constants of these complexes.
RESULTS AND DISCUSSION
Chemical literature26),(27 confirms that anionic species of amino acids are the sole ligating species for metal ions. The electrophoretic mobility of the metal spot against pH gives a curve with number of plateaus is shown in Figure 1.
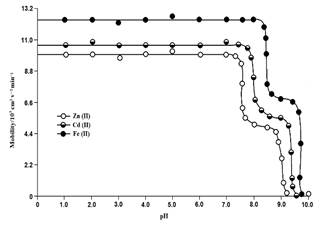
Figure 1 Mobility curves for the metal (II) - sarcosine complexes. Background electrolytes = 0.1 mol L-1 perchloric acid and 0.01 mol L-1 sarcosine. Concentration of Cd (II), Fe (II) and zinc (II) = 0.005 Mol L-1. Variation in the pH was made by the addition of sodium hydroxide.
A constant speed over a range of pH is possible only when a particular complex species is overwhelmingly formed. Thus, every plateau is indicative of formation of a certain complex species. The first one in the beginning corresponds to a region in which metal ions are uncomplexed. In this region of low pH, concentration of unprotonated species of sarcosine [CH3(NH2 +) CH2 COOH] is maximum and this range, metals ions spots possess progressively decreasing mobility, complexation of metal ions should be taking place with anionic species of sarcosine, whose concentration increases progressively with increase of pH. Figure 1 shows three plateaus in Cd (II), Fe (II) and Zn (II) hence all three metal ions form two complexes with sarcosine anion. Prominent chelating properties have been assigned to unprotonated anionic species sarcosine, ruling out any such property to the zwitterions28.
Figure 1 discloses that Cd (II), Fe (II) and Zn (II) metal ions form their first complex movement towards negative electrode. Hence, one anionic species of sarcosine, [CH3 - (NH) - CH2 - COO-], must have combined with Cd (II), Fe (II) and Zn (II) metal ions to give 1:1, [Cd{CH3 - (NH) - CH2 - COO}]+, [Fe{CH3 - (NH) - CH2 - COO}]+ and [Zn{CH3 - (NH) - CH2 - COO}]+, complex cations, respectively. The third plateau in each case is due to 1:2 metal-ligand complex. Hence, two anionic species of sarcosine [CH3 - (NH) - CH2 - COO-], must have combined with Cd (II), Fe (II) and Zn (II) metal ions to give 1:2 [Cd {CH3 - (NH) - CH2 - COO}2], [Fe {CH3 - (NH) - CH2 - COO}2], [Zn{CH3 - (NH) - CH2 - COO}], neutral metal complexes, respectively.
Further increase of pH has no effect on mobility of metal ions and ligands. The complexation of metal ions with sarcosine anion may be represented as
where, M2+ = Cd2+, Fe2+ and Zn2+ metal cations; [L-] = sarcosine anion; K1 and K2 are the first and second stability constants, respectively. The metal spot on the paper is thus a combination of uncomplexed metal ions, 1:1 and 1:2 metal complexes. The spot is moving under the influence of electric field, if non-protonated and protonated pieces are considered, the overall mobility can be given by expression29.
where, u0, u1 and u2 are the same mobilities of uncomplexed metal ions, 1:1 complex and 1:2 complexes, respectively. The protonation constants of pure sarcosine (pk1 = 2.20; pk2 =9.99) were obtained by the paper electrophoretic technique. The mode of dissociation of pure sarcosine can be represented as:
Using protonation constants of pure sarcosine, the concentration of norvaline anion [L-] is determined for pH value (s) of interest, from which K1, can be calculated. The concentration of complexing sarcosine anion [L-] is calculated with the help of equation.
where, [LT] = total concentration of ligand sarcosine [0.01 Mol L-1]; pka1 and pka2 = first and second protonation constant of pure sarcosine, respectively.
For calculating first stability constant K1, the region between first and second plateau is pertinent. The overall mobility will be equal to the arithmetic mean of the mobility of uncomplexed, u1, at a pH value where K1 = 1/ [CH3- (NH) - CH2COO-].
The second stability constant K2 of 1: 2 complex can be calculated by taking into consideration, the region between the second and third plateau of mobility curve. The (se) calculation values of K1 and K2 are given in Table 1.
Table 1 Stability constants of binary complexes of cadmium (II), iron (II) and zinc (II) with sarcosine
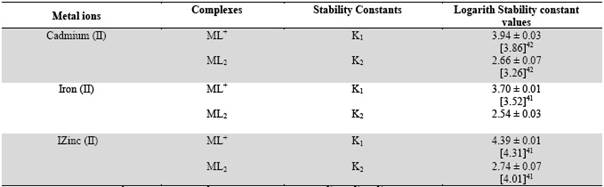
Ionic strength = 0.1 Mol L-1; temperature = 350 C; M = metal cations (Cd2+, Fe2+ , Z2+); L = ligand (sarcosine); sarcosine anion; [CH3 - (NH) - CH2 - COO-]; * Literature values are given in the bracket.
It is clear from Table 1 that first and second stability constants follow the order: zinc (II)> cadmium (II) > iron (II) the second stability constant values are found to be lower in comparison to first stability constant in each case, this may be due to the decrease in coordinating tendency of ligand with higher state of aggregation30. It is also clear from Table 1 that the calculated stability constant values are similar to literature values. The slight deviation in the values obtained from different sources is mainly due to the difference in temperature and ionic strength used by different workers.
High stability constant values of zinc (II) - sarcosine complexes indicate strong bonding between zinc (II) cation and sarcosine anion. Whilst low stability constant values of iron (II) complexes indicate weak bonding between iron (II) cation sarcosine anion. The higher stability of zinc (II) complexes may be ascribed to its greater affinity for oxygen donor ligands. The stability constants of metal complexes can be very easily calculated by this technique, therefore present method has significant advantages over other methods reports in chemical literature for the determination of stability constants of metal complexes (viz: polarography, potentiometry, solubility etc.). According to standard deviation (statistics) the precision of the method is limited to that of paper electrophoresis, and uncertainty in the result is ± 5 %. Hence, it cannot immediately replace the most reliable methods, even though it is new approach deserving further development.
The recent parallel studies on metal complexes in biology and medicine has been reported in chemical literature. Polaprezinc, a chelated form of zinc and L - carnosine is a new generation gastric mucosal protective agent that has been used in clinical for more than 20 years in Japan31. Fe has many oxidation states ranging 2- to 6+, but 2+ (d6) and 3+ (d5) oxidation states ranging or of the greatest importance in biological systems and exquisitely sensitive to both pH and the nature of ligating functionality32. The summary of proper treatment of Cd poisoning, based on the use of selected Cd destroying agents and chelators, and the potential preventive approaches to counteract it chronic exposure is found in the literature33. In vitro antibacterial and antitumor potential of iron based on Schiff bases is described by Aly et al.34 Physiological and pharmacological applications of antioxidant activity of ferrozine - iron - amino acid complexes is reported by Berlette et al.35. A complex of Zn and carnosine called Zinc - L - Carnosine (Zn C) has been extensively used in tumor adjuvant therapy36. Cd is toxic metal for human, organisms and for all ecosystems. The long biological half-life of Cd makes it a cumulative toxin, chronic exposure and causes harmful effects from the metal stored in the organs37. Synthesis of Cd complexes using N (4) - phenyl - 2 - formylpridine thiosemicarbazone (L1) and 5 - aminotetrazole (L2) as organic ligands and evolution of their anti - cancer and nephrotoxic potential in vitro is reported by Abyar et al.38. A novel 5- nitro - 8 - hydroxyquinoline - proline hybrid and its Rh (n5 - C5 Me5) and Ru (n6 - p - cymene) complexes with excellent aqueous solubility were developed characterized against sensitive and multiple drug resistance cells39.
CONCLUDING REMARKS
Cadmium (II), iron (II) and zinc (II) are significant for biological systems but as such they are toxic, the sarcosine may be used to reduce the level of these metal ions in the biological system. Zinc (II) - sarcosine complexes have high stability constant values in comparison to iron (II) - sarcosine and cd (II) - sarcosine complexes. The present paper electrophoresis technique is very helpful in finding that complex systems is formed or not, if formed its stability constant can also be determined. The ML2 complexes are found to have low stability constant values and less stable in comparison to ML complexes. Stability constants of metal complexes can be very easily calculated by this technique, so the present paper electrophoretic technique has significant advantages over the other physiochemical methods reported in chemical literature for the determination of stability constants of metal complexes.
EXPERIMENTAL
Apparatus
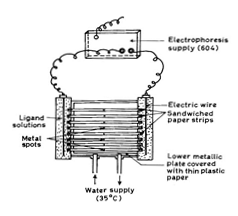
A Systronic (Naroda, India) Model 604 electrophoresis system was used. The apparatus consisted of a poly (vinyl chloride) PVC moulded double tank vessel. In our laboratory significant change in the instrument has been made. Two hollow rectangular iron plates each weighing one kg and covered with thin polythene sheets have been used through which thermostatic water circulated for controlling the temperature. The tanks are closed with a transparent PVC moulded lid. The whole assembly is tight to prevent moisture changes, which might upset the equilibria in the paper strip. The assembly design thus keeps to a minimum the disturbing effects of evaporation from unwanted liquid flow in the paper strip. Each electrolyte tank contains a separate electrode chamber in which Pt-wire anode and cathode are placed, respectively. Applied voltage was from a stabilized source. Electrophoresis cell showing sandwiched paper strips is shown in Figure 2.
Whatman No. 1 filter paper for chromatography were used for the purpose of electrophoresis. Elico (Hyderabad, India), Model L1-10, pH meter using a glass and calomel electrodes assembly working on 220 V/50 Hz established a. c. mains, was used for the pH measurement. pH meter was calibrated with buffer solution of pH 7.0.
Chemicals
Metal Solutions
Solutions of cadmium (II), iron (II) and zinc (II) perchlorate were prepared by preliminary precipitation of metal carbonates from 0.1 M solution of sodium carbonate (chemically pure grade BDH, Poole, (UK) were washed with boiling water and treated with calculated amounts of 1 % Analytical Regent Grade per chloric acid. These were boiled on a water bath and filtered. The metal contents of the filtrates were determined, and the final concentration was kept at 5.0 x 10-3 M40.
Detecting reagent for metal ions and glucose
Metal spots were detected on the paper using dithizone in carbon tetrachloride for Zn (II). A 0.1% solution of 1 - (2 - pyridylazo) -2- naphthol (PAN) (E.Merck, Darmstadt, Germany) in ethanol was used for detecting the cadmium (II) and iron (II) metal ions. A 0.005 M glucose (BDH, AnalaR) solution were prepared in water and used as an electro-osmotic indicator for the correction due to electro-osmosis.
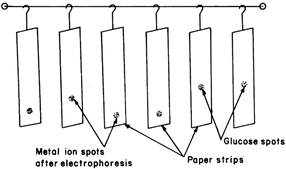
A saturated aqueous solution (0.9 mL) of silver nitrate was diluted with acetone to 20 mL. Glucose was detected by spraying with this silver nitrate solution and then with 2 % ethanolic sodium hydroxide, when a black spot was formed. Paper strips showing position of metal ion spots and glucose spots after electrophoresis is shown in Figure 3.
Background electrolytes (BGEs)
Stock solution of 5.0 mol L-1perchloric acid was prepared by is 70% solution (SDS, AnalaR grade), 0.5 mol L-1 sarcosine (BDH, Poole, UK) and 2.0 mol L-1 sodium hydroxide (Analytical - Reagent grade) solutions were prepared. The background electrolytes were in the study of binary complexes were 0.1mol L-1 perchloric acid and 0.1 mol L-1sarcosine. The binary system was maintained at various pHs by the addition of sodium hydroxide.
Procedure
Binary complexes
For recording observation of particular metal ion, two paper strips were spotted with metal ion solution along with additional two spotted with glucose using 1.0 µL pipette and then mounted on the insulated plate. The hallow base plate in the instrument was made horizontal using a spirit level and 150 - mL volume of BGEs containing 0.1 M per chloric acid and 0.01 M sarcosine was placed in each of the two tanks of the electrophoretic apparatus. The paper became moistened with the BGEs solution due to diffusion. The second insulated plate was placed on paper strips and then thermostated water (350 C) was circulated in the plates to keep the temperature constant. The lid was then placed on the instrument to make it airtight. It was left for 15 minutes to insure wetting of the strips. Subsequently 200 V potential difference was then applied between the tank solutions to initiate electrophoresis. The electrophoresis was carried out for 60 minutes after which, the paper strips were taken out by means of glass rods, dried on a horizontal platform and spots detected. The observations were repeated for different pH values of BGE (variation in pH was made by addition of sodium hydroxide solution). The differences in the distances recorded in the duplicates were within ± 5 % and the average distances in the duplicates were noted for the calculation. The distance travelled toward the anode was assumed to be negative and that toward cathode positive. The actual distance of the sample spot was measured after taking into account the distance travelled by the reference glucose spot.
Ionophoretic observation of metal ions were recorded at various pH values of the BGE obtained by adding sodium hydroxide solution, the ionic strength being maintained at 0.1 M. the observed mobility of migrant was calculated by using the formula.
After applying the correction factor the observed mobility is given as:
where U = mobility of metal ion / complex ion; d = mean of duplicate distance travelled by metal ion / complex ion; dG = mean of duplicate distance travelled by glucose spot; x = field strength; t = time for electrophoresis.
The protonation constants of pure sarcosine were determined by using the same paper electrophoresis technique. For recording the mobility of sarcosine two paper strips were spotted with sarcosine along with two other spotted with glucose using 0.1 M per chloric acid only as the BGE. The electrophoresis was carried out for 60 minutes as for metal ions. The electrophoretic mobility was calculated.












 uBio
uBio