Journal of the Selva Andina Animal Science
versión impresa ISSN 2311-3766versión On-line ISSN 2311-2581
J.Selva Andina Anim. Sci. vol.11 no.1 La Paz 2024 Epub 01-Abr-2024
https://doi.org/10.36610/j.jsaas.2024.110100013
ARTÍCULOS DE INVESTIGACIÓN
Indicators of kidney function in guinea pigs (Cavia porcellus) fed with the inclusion of pisonay (Erythrina edulis) meal of three regrowth ages
1Micaela Bastidas National University of Apurimac. Faculty of Veterinary Medicine and Animal Husbandry. Laboratory of Pharmacology, Toxicology and Veterinary Biochemistry. Corner of Los Lirios and Alamos Streets. Abancay, Apurímac, Peru.
El objetivo del estudio fue determinar los niveles séricos de creatinina, nitrógeno ureico en sangre (NUS) y la relación riñón peso vivo de cuyes (Cavia porcellus) alimentados con la inclusión de harina de pisonay (Erythrina edulis) de 3 edades de rebrote en el sector de Mosoccpampa, Apurímac. Se utilizaron 80 cuyes machos mejorados, que fueron distribuidos al azar en grupos de 8 cuyes para cada tratamiento dietético que contenía 10, 20 y 30 % de inclusión de harina de pisonay (HP) por cada edad de rebrote de 4, 8 y 12 meses y un grupo control con 20 % de harina de alfalfa en condiciones isoprotéicas e isoenergéticas. Se tomaron muestras de sangre con finalidad de determinar la actividad sérica mediante kits comerciales (Valtek Diagnostics) para fotometría. Los datos fueron analizados bajo el diseño completamente al azar y para la comparación de medias se aplicó el contraste de Dunnett’s (p≤0.05). Los niveles séricos de creatinina de las dietas D2, D3, D4 y D8 (0.61, 0.68, 061 y 0.66 mg dL-1 respectivamente) fueron diferentes al grupo control 0.48 mg dL-1 (p<0.05). Los niveles de NUS de las dietas D1 21.26 mg dL-1 y D7 14.05 mg dL-1 fueron diferentes al grupo control 17.56 mg dL-1 (p<0.05). La relación riñón peso vivo en las dietas (0.85 %) fueron similares a la dieta control 0.87 % (p>0.05). Los valores de creatinina sérica, NUS y la relación riñón peso vivo no fueron afectados por la inclusión de HP de 3 edades de rebrote en la dieta, esté comportamiento nos indicaría que la HP podría ser considerado como insumo para elaborar alimento integral para cuyes en la etapa de crecimiento.
Palabras clave: Creatinina; cuyes machos; hojas; nitrógeno ureico en sangre; valle interandino
The objective of the study was to determine the serum levels of creatinine, blood urea nitrogen (BUN) and the kidney weight ratio of guinea pigs (Cavia porcellus) fed with the inclusion of pisonay (Erythrina edulis) meal of three regrowth ages in the Mosoccpampa sector, Apurímac. Eighty improved male guinea pigs we´re used, which we´re randomly distributed into groups of 8 guinea pigs for each dietary treatment containing 10, 20 and 30 % inclusion of pisonay meal for each regrowth age of 4, 8 and 12 months and a control group with 20 % alfalfa meal under isoproteic and isoenergetic conditions. Blood samples we´re taken to determine serum activity using commercial kits (Valtek Diagnostics) for photometry. The data we´re analyzed under the completely randomized design and for the comparison of means, the Dunnett´s test was applied (p≤0.05). Serum creatinine levels of the D2, D3, D4 and D8 diets (0.61, 0.68, 061 and 0.66 mg dL-1 respectively) were different from the control group (0.48 mg dL-1) (p<0.05). BUN levels of the D1 (21.26 mg dL-1) and D7 (14.05 mg dL-1) diets were different from the control group (17.56 mg dL-1) (p<0.05). The kidney weight ratio in the diets (0.85 %) was similar to control diet (0.87 %) (p>0.05). The values of serum creatinine, BUN and kidney weight ratio we´re not affected by the inclusion of pisonay meal of three regrowth ages in the diet, this behavior would indicate that pisonay meal could be considered as input to produce integral food for guinea pigs in the growth stage.
Keywords: Blood urea nitrogen; creatinine; interandean valley; leaves; male guinea pigs
Introduction
Guinea pig meat is an alternative for consumption with respect to other types of meat. It’s being successfully raised on the coast and high Andean areas of Peru, they are a source of income for producers, and the feeding of guinea pigs can vary, from the use of whole food on the coast, and in the highlands the use of alfalfa or other fodder, or a combination of both1. The national guinea pig population in 2012 was estimated at 1 012 181 and a per capita consumption of 0.35 kg/inhabitant/year2, in 2017 the guinea pig population in Peru was 17 380 000 animals3 and is currently mentioned above 20 million, the Apurímac region would rank fourth in number of guinea pigs.
The genus Erythrina contains in its leaves alkaloids, flavonoids, terpenoids4, tannins and total polyphenols5, antinutritional factors, which can cause cytotoxic effects6, and could affect the weight of internal organs. The foliage of trees, shrubs and bushes in hay and ground has shown its nutritional and productive qualities as an input in the production of concentrated feed for guinea pigs7,8. Such as the leaves of E. edulis (pisonay), with a nutritional composition of the fresh forage (wet basis), with respect to dry matter (DM) 25.39 %, crude protein (CP) 7.65 %, crude fiber 7.35 %, crude fat 0.31 % and ash 1.29 %9. Also, in leaves and petioles of Erythrina sp., pruned from trees used for animal feed, DM ranged from 24.8 to 31.7 %, CP from 20.1 to 23.5 %, crude fiber 7.35 %, crude fat 0.31 % and ash 1.29 %. Ethereal extract from 0.4 to 2.5 %, ash from 8.6 to 11.6 % neutral detergent fiber was most stable at 58.0 % and acid detergent fiber from 32.6 to 34.7 %10,11. The inclusion of forages and non-conventional feedstuffs such as meal in the guinea pig diet did not modify the productive behavior or the quality of the feed12. Erythrina leaves as meal in guinea pig feed caused variations in the profiles of total protein, blood albumin and carcass yield13. Domestic rodents with signs of renal insufficiency, high levels of BUN and creatinine serum were observed14. In addition, in guinea pigs with renal functional impairment was manifested by a doubling of creatinine concentration serum and increased of BUN15. In degenerative and metabolic diseases affecting the urinary system are so far analyzed in the context of laboratory rodents16.
Biochemical studies and the analysis of organ weight through the organ-body weight ratio are important to evaluate the toxicity of antinutritional factors, in rabbits fed with the inclusion of Gliricidia sepium leaf meal in proportions of 10 and 15 % did not cause variations in the values of internal organs, expressed as a percentage of live weight17. Mucuna utilis in graded levels did not present a significant difference (P>0.05) in the weight of organs18. 20 % of Moringa oleifera in the dietary intake of caused a downward trend in live weight and kidney weight19. In this sense, due to the nutritional benefits, the use in animal feed and possible toxicity of pisonay, our objective was to determine the serum levels of creatinine, BUN and the live weight kidney ratio of guinea pigs (C. porcellus) fed with the inclusion of pisonay meal at three different ages of regrowth.
Materials and methods
The work was carried out in a guinea pig shed, built with adobe material and an area of 200 m2, located in Mosoccpampa, Tamburco, at an altitude of 2800 masl, the minimum and maximum temperatures were 6.8 and 23.7° C, the annual rainfall was 1022 mm and the relative humidity was 73.6 %. In addition, biosecurity protocols we´re applied to prevent diseases in the guinea pigs20.
The guinea pigs were from the La Inmaculada farm (commercial breeding) located in Curahuasi, which it registered in the Regional Directorate of Agriculture of Apurimac, 80 type I male guinea pigs of approximately 15 days of age we´re used, with an average live weight of 324.05±37.23 g, which we´re randomly distributed to 10 experimental groups.
The guinea pigs we´re raised in one-story cages with an area of 0.81 m2, the living space for each guinea pig was (0.20 m2)21, the cages we´re provided with hopper type feeders with a metal base and corduroy type drinkers.
The leaves and petioles we´re harvested by pruning pisonay (E. edulis) trees located from area; the characteristics observed in the trees we´re described by Cárdenas-Villanueva22. Normally used for animal feed as fresh forage, pruning was carried out according to the last cut for each age of regrowth (4, 8 and 12 months), the foliage was subjected to shading for approximately 30 days, after which it was milled in a hammer mill with a 2 mm sieve.
Nine balanced diets were prepared under isoproteic and isoenergetic conditions (Table 1), 3 for each age of pisonay regrowth and 1 control diet with the inclusion of alfalfa meal (D0). The diets were prepared using wheat bran, alfalfa meal (20 %) and pisonay meal (10, 20 and 30 %), ground corn, soybean cake, and the following additives: dicalcium phosphate, calcium carbonate, common salt, vitamin C, myco- sequestering agent, premix (vitamins and minerals) and DL-Methionine, which we supplied by Del Corral enterprise.
Table 1 Calculated nutritional composition of diets for guinea pigs
| Nutrients | D0 | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Dry matter, % | 93.4 | 93.7 | 93.6 | 93.5 | 93.6 | 93.6 | 93.5 | 93.6 | 93.6 | 93.8 |
| Crude protein, % DM | 17.4 | 17.9 | 17.7 | 17.5 | 17.8 | 17.7 | 17.6 | 17.9 | 17.8 | 17.8 |
| Ethereal extract, % DM | 1.8 | 2.3 | 2.0 | 1.9 | 2.3 | 2.1 | 1.9 | 2.4 | 2.3 | 2.3 |
| Ash, % DM | 4.3 | 4.6 | 4.8 | 5.1 | 4.6 | 4.9 | 5.4 | 4.6 | 5.2 | 5.6 |
| Neutral detergent fiber, % DM | 32.3 | 36.0 | 33.6 | 31.7 | 36.0 | 33.9 | 32.0 | 36.1 | 34.2 | 32.6 |
| Digestible energy, Mcal/kg | 3.06 | 2.96 | 3.01 | 3.01 | 2.97 | 3.01 | 3.01 | 2.98 | 2.98 | 3.01 |
The diets we fed in meal, 7 days in the habituation phase and 56 days in the experimental phase, in each phase the clinical status of the animals was evaluated, the guinea pigs were fed without restriction once a day and fresh water was offered ad libitum.
After the conclusion of the experimental phase (56 days) all the guinea pigs we benefited, the blood of each guinea pig was collected directly from the jugular vein in test tubes without anticoagulant.
Previously the guinea pigs were insensitized23, then the blood of each guinea pig was centrifuged (Hettich Rotofix 32A) to obtain blood serum, which was transferred to 5 mL vials and frozen at -20° C (Boch no frost freezer), and then creatinine and BUN were determined by means of a semi-automated biochemical analyzer (Stat Fax 3300).
To determine the serum levels of creatinine and BUN, 2 repetitions were performed for each blood serum sample from all guinea pigs, using the procedures proposed by the commercial firm Valtek Diagnostics, Chile, containing working reagent and standard or calibrator.
For the kidney live weight ratio, the guinea pigs were weighed at the end of the experiment on a Henkel BRD04kF digital balance (± 1.0 g). After the benefit, the kidneys of each of the guinea pigs were extracted and weighed on an Ohaus Ad- venturer AX5202 analytical balance (± 0.01 g), and the ratio was determined according to the following formula:
To perform the analysis of variance, these percentage values, being below 30 %, were subjected to the angular or Bliss transformation.
The normality of the serum levels and the live weight-ratio of each of the diets was evaluated by the d'Agostino-Pearson test, the data were analyzed under a completely randomized design and for the comparison of means the Dunnett's test was applied (p≤0.05), previously the test of homogeneity of variances was performed through Levene's test. In addition, the linear correlation between the 3 variables was performed.
Results
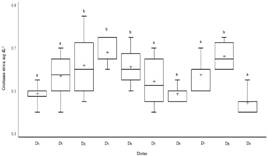
The serum creatinine levels (Figure 1) of the diets D2 (0.61±0.14 mg dL-1), D3 (0.68±0.05 mg dL-1), D4 (0.61±0.08 mg dL-1) and D8 (0.66±0.20 mg dL-1) were different to D0 (0.48±0.04 mg dL-1) (p<0.05). In diet D2 the highest extreme value of 0.85 mg dL-1 was observed in several diets the lowest value was 0.40 mg dL-1.
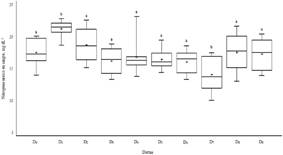
The BUN levels (Figure 2) of the diets D1 (21.26±1.33 mg dL-1) and D7 (14.05±2.78 mg dL-1) were different from the control diet (17.56±2.15 mg dL-1) (p<0.05). The most extreme value was observed in diet D4 (23.15 mg dL-1) and the lowest value was in D7 (10.00 mg dL-1).
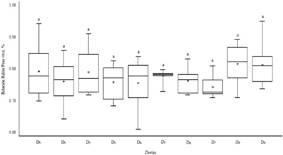
The effect of the diets with the inclusion of pisonay meal was not significant (p>0.05) in the live weight kidney ratio (Figure 3) of guinea pigs with respect to the D0 diet (0.87 %). The most extreme value was observed in diet D9 (1.02 %) and the lowest value was in D4 (0.74 %). The correlation between the variables creatinine and live weight kidney ratio (Table 2) was significant (p<0.05), which suggests that there is a negative linear relationship and the strength of the linear correlation was (r: ±0.09 to ±0.00) between BUN and kidney live weight ratio (p>0.05).
Table 2 Linear correlation between study variables
| Creatinine, mg dL-1 | BUN, mg dL-1 | ||
|---|---|---|---|
| Creatinine, mg dL-1 | .1047 p = .3554 | -.2407 p = .0315 | |
| BUN, mg dL-1 | .1047 p = .3554 | -.0387 p = .7335 | |
| K/LW Ratio, % | -.2407 p = .0315 | -.0387 p = .7335 |
BUN: Blood urea nitrogen. K/LW: Kidney live weight ratio. p: probability.
Discussion
With respect to serum creatinine, the values by effect of diets D2, D3, D4 and D8 would be close to the lower limit of 0.6 mg dL-1 proposed by Gross24. All diets remained in the ranges (0.1 to 0.9 mg dL-1) in 13/N hybrid male guinea pigs25. Diets D0, D6 and D9 remained between 0.33 to 0.51 mg dL-1 values in Weiser-Maples male guinea pigs26 and the serum creatinine levels due to the effect of the diets would not be in the range (0.75 to 2.55 mg dL-1) referential for albino guinea pigs27, these variations would probably be due to the species under study and age.
This amplitude in the ranges for creatinine suggests that the inclusion of pisonay meal from 3 ages of regrowth would not cause significant changes or toxicological implications. As occurred with pisonay supplied to guinea pigs as fresh fodder above 50 % which increased serum creatinine levels above (3.1 mg dL-1)28. Also Lantana cámara as a non-conventional fodder, which is consumed in times of scarcity, increased creatinine levels (1.21 mg dL-1) with respect to the control group (0.54 mg dL-1)29. With vegetable-based diets caused creatinine to reach up to 4.2 mg dL-1 probably due to the presence of oxalates30 and with mixed feeding, fresh alfalfa was included, offered there was no evidence of renal damage in male guinea pigs31. This increase in serum creatinine could be related to mild exposure to nephrotoxic substrates that would cause subchronic toxicity29,32 in a given time to chronic kidney disease where 75 % of renal function is lost33. The BUN due to the effect of the diets was similar to the range of 17 to 29 mg dL-1 for 13/N hybrid male guinea pigs25. With the exception of D7 and in all cases was in the range (12.5 to 41.8 mg dL-1) referential for albino guinea pigs27. Opposite occurs when contracted with the range (26.6 to 37.3 mg dL-1) found in Weiser-Maples male guinea pigs26.
The reported values of BUN were similar to the values with the inclusion of fresh forage from pisonay in the diet for guinea pigs where it was obtained from (12.8 to 16.8 mg dL-1)28. Diets based on vegetables in the guinea pig diet, a double increase was observed with respect to the normal value for BUN, probably due to the ingestion of vegetables containing oxalate30. These variations in the values found, would be insufficient to assert the presence of renal disease, the BUN would increase from 15 to 40 times in guinea pigs34 as would occur in renal failure33. The inclusion of pisonay meal up to 30 % would not result in nephrotoxicity, this anomaly would be subject to adult guinea pigs, young immunocompromised or physiologically stressed guinea pigs35.
The inclusion of pisonay meal did not result in high levels of creatinine serum and BUN. These values are in the normal reference range for guinea pigs, which would indicate that glomerular filtration and secretion in the proximal tubule would be normal and would not cause acute renal damage36. On the other hand, we could assume that the extent of renal damage was tolerable in the initial and experimental phase since no mortality was observed in the guinea pigs.
The kidney/live weight ratio remained stable as the percentage of pisonay meal of each regrowth age increased. Being congruent with the observation in apparently healthy adult male guinea pigs, reared in captivity and fed with food pellets, carrots and fruits only, which had a ratio of 0.88%37. Similarly, the inclusion of 50 and 75 % of potato plants in the diet of guinea pigs, after 54 days of experimentation, the ratio decreased from 0.90 to 0.80 % respectively (p<0.05)38. Is contradictory, when compared with male guinea pigs of the Peru line that received ground flaxseed (100 g) in isonitrogenous concentrates plus alfalfa for 30 days, they showed a ratio of 1.02 %39. In another study, the weight of gastrointestinal tract, kidneys and lungs (g/100 g of body weight) of male guinea pigs of the Peru breed, after 42 days, had a slight decrease due to the effect of diets with different dietary electrolyte balance, which also moderately increased the levels of creatinine and BUN40.
This is corroborated by the final live weight observed the guinea pigs achieved 1032.38±111.08 g and with the control diet they reached weights of 1031.50±167.81 g. This behavior can be associated to the adequate protein level of the diets that did not cause an increase in the value of the BUN41, which remained within the normal range for guinea pigs. Furthermore, the similarity in body weight would be related to the physiological response of adaptation to the possible toxic effects, which did not cause a reduction in food consumption and therefore in the absorption of nutrients42.
The negative linear correlation observed between creatinine and Rel/PV ratio (p<0.05) and the absence of significant difference in Rel/PV ratio, would indicate that the relative weight of vital organs such as kidneys would be in normal conditions43, therefore, there would be no toxic effects due to the inclusion of HP in the diet of growing guinea pigs.
REFERENCES
1. Huamaní G, Zea O, Gutiérrez G, Vílchez C. Efecto de tres sistemas de alimentación sobre el comportamiento productivo y perfil de ácidos grasos de carcasa de cuyes (Cavia porcellus). Rev Inv Vet Perú 2016;27(3):486-94. DOI: http://dx.doi.org/10.15381/rivep.v27i3.12004 [ Links ]
2. Instituto Nacional de Estadistica e Informatica. Resultados definitivos. IV censo nacional agropecuario 2012 ENT#091;InternetENT#093;. Lima: Instituto Nacional de Estadistica e Informatica; 2013 ENT#091;citado 12 de marzo de 2023ENT#093;. 63 p. Recuperado a partir de: https://proyectos.inei.gob.pe/web/DocumentosPublicos/ResultadosFinalesIVCENAGRO.pdf [ Links ]
3. Instituto Nacional de Estadistica e Informatica. Encuesta Nacional Agropecuaria 2017 ENT#091;InternetENT#093;. Lima: Instituto Nacional de Estadistica e Informatica; 2018 ENT#091;citado 10 de mayo de 2023ENT#093;. Recuperado a partir de: https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1593/ [ Links ]
4. Pino-Rodriguez S, Prieto-González S, Pérez-Rodriguez ME, Molina-Torres J. Género Erythrina: Fuente de metabolitos secundarios con actividad biológica. Acta Farm Bonaer 2004;23 (2):252-8. [ Links ]
5. Ramirez-Borda Y, Cárdenas-Villanueva LA, Ramos-De la Riva VA, Gómez-Quispe OE. Serum concentration of aminotranferases in guinea pigs (Cavia porcellus) fed diets based on pisonay (Erythrina sp). Rev Investig Vet del Perú 2019;30 (3):1099-108. DOI: http://dx.doi.org/10.15381/rivep.v30i3.16604 [ Links ]
6. Araújo-Júnior JX, Oliveira MSG, Aquino PGV, Alexandre-Moreira MS, Sant'Ana AEG. A Phytochemical and ethnopharmacological review of the genus Erythrina. In: Rao V, editor. Phytochemicals - A Global Perspective of Their Role in Nutrition and Health. Londres: Intecho-pen; 2012. p. 327-52. DOI: https://doi.org/10.5772/26997 [ Links ]
7. Guevara J, Díaz P, Bravo N, Vera M, Crisóstomo O, Barbachán H, et al. Use flour pajuro (Erythrina edulis) as food supplement in guinea pig-Lima. Rev Per Quím Ing Quím 2013;16(2):21-8. [ Links ]
8. Meza GA, Loor NJ, Sánchez AR, Avellaneda JH, Meza CJ, Vera DF, et al. Leaf meals and tropical shrubby foliage (Morus alba, Erythrina poeppigiana, Tithonia diversifolia and Hibiscus rosasinensis) in feeding guinea pigs (Cavia porcellus Linnaeus). Rev Fac Med Vet Zoot 2014;61(3): 258-69. DOI: https://doi.org/10.15446/rfmvz.v61n3.46874 [ Links ]
9. Huarcaya Miraya MG. Las hojas y frutos del antiporoto (Erythrina edulis) en la alimentación animal en Kerapata Tamburco Abancay 2018 ENT#091;tesis licenciaturaENT#093;. ENT#091;AbancayENT#093;: Universidad Tecnologica de los Andes; 2020 ENT#091;citado 26 de mayo de 2023ENT#093;. Recuperado a partir de: https://repositorio.utea.edu.pe/handle/utea/260 [ Links ]
10. Cárdenas-Villanueva LA, Bautista-Pampa JL, Zegarra-Paredes JL, Ramos-Zuniga R, Gómez-Quispe OE, Barreto-Carbajal JS. Degradabilidad in situ de la materia seca y proteína cruda de las hojas y peciolo del pisonay (Erythrina falcata). Rev Investig Vet del Perú 2016;27(1):39-44. DOI: http://dx.doi.org/10.15381/rivep.v27i1.11461 [ Links ]
11. Choque Durant H, Huaita Patiño A, Cárdenas Vilanueva LA, Ramos Zuñiga R. Effect of regrowth age the ruminal degradation of pisonay (Erythrina sp) in Andean valley of Abancay. Rev Investing Altoandin 2018;20(2):189-202. DOI: http://dx.doi.org/10.18271/ria.2018.363 [ Links ]
12. Apraez-Guerrero JE, Fernández-Pármo L, Hernández-Gonzáles A. Effect of the usage of grasses and non conventional feed on the productive behavior, carcass performance and meat quality of guinea pigs (Cavia porcellus). Veterinaria y Zootecnia 2008;2(2):29.34. [ Links ]
13. Paredes-López D, Robles-Huaynate R, Córdova-Chumbes O, De la Cruz-Paucar E. Effect of the Erythrina sp. leaves powder on biochemical profile, biological parameters and liver histopathology of Cavia porcellus. Scientia Agropecuaria 2017;8(4):297-304. DOI: https://dx.doi.org/10.17268/sci.agropecu.2017.04.01 [ Links ]
14. Johnson-Delaney CA. Disease of the urinary system of commonly kept rodents: Diagnosis and treatment. Semin Avian Exot Pet Med. 1998;7(2):81-8. DOI: https://doi.org/10.1016/S1055-937X(98)80046-7 [ Links ]
15. Couser WG, Stilmant MM, Jermanovich NB. Complement independent nephrotoxic nephritis in the guinea pig. Kidney Int 1977;11(3):170-80. DOI: https://doi.org/10.1038/ki.1977.25 [ Links ]
16. Kohn RA, Dinneen MM, Russek-Cohen E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J Anim Sci 2005;83(4):879-89. DOI: https://doi.org/10.2527/2005.834879x [ Links ]
17. Edoh JH, Annongu AA, Houndonougbo FM, Houndonougbo PV, Ajide SO. Assay of detoxification potential of DL-Methionine on dietary Gliricidia leaf meal in rabbit nutrition: relative organ weight and blood indices. Int J Adv Res Publ 2019;3(4):47-53. [ Links ]
18. Sese BT, Okpeku M, Igirigi A. Impact of tropical velvet bean (Mucuna utilis) leaf meal on performance, organ weight and haematological indices of young rabbits. J Anim Sci Adv 2014;4(4):777-86. [ Links ]
19. Ghomsi MOS, Enow JT, Etchu KA, Tientcheu BL, Enamou G, Chouengouong TM, et al. Effect of Moringa Oleifera Leaf Meal (Molm) on the growth, carcass, heamatology and biochemical parameters of rabbits. SOJ Vet Sci 2017;3(3):1-5. DOI: https://doi.org/10.15226/2381-2907/3/3/0013 [ Links ]
20. Huamán Alcánta M. Manual de bioseguridad y sanidad en cuyes ENT#091;InternetENT#093;. Lima: Instituto Nacional de Innovación Agraria; 2019 ENT#091;citado 20 de febrero de 2022ENT#093;. Recuperado a partir de: https://repositorio.inia.gob.pe/bitstream/20.500.12955/936/1/Huamán-Manual_de_Bioseguridad_y_Sanidad_en_cuyes.pdf [ Links ]
21. Mínguez Balaguer C, Calvo Capilla A, Zeas Delgado VA, Sánchez Macías D. A comparison of the growth performance, carcass traits, and behavior of guinea pigs reared in wire cages and floor pens for meat production. Meat Sci 2019;152(6):38-40. DOI: https://doi.org/10.1016/j.meatsci.2019.02.012 [ Links ]
22. Cárdenas Villanueva LA. Valor nutricional del pisonay (Erythrina edulis) en cuyes (Cavia porcellus) ENT#091;tesis doctoralENT#093;. ENT#091;PunoENT#093;: Universidad Nacional del Altiplano; 2022. Recuperado a partir de: https://repositorio.unap.edu.pe/handle/20.500.14082/19442 [ Links ]
23. Jurado-Gámez HA, Cabrera-Lara EJ, Salazar Salazar JA. Comparison of two types of sacrifice and different ripening times on physico-chemical and microbiological variables of guinea pig (Cavia porcellus) meat. Rev Med Vet Zoot 2016;63(3):201-17. DOI: https://doi.org/10.15446/rfmvz.v63n3.62741 [ Links ]
24. Gross DR. General principles of animal selection and normal physiological values. In: Gross DR, editor. Animal Models in Cardiovascular Research. New York: Springer Nature; 2009. p. 1-15. DOI: https://doi.org/10.1007/978-0-387-95962-7_1 [ Links ]
25. Genzer SC, Huynh T, Coleman-McCray JD, Harmon JR, Welch SR, Spengler JR. Hematology and clinical chemistry reference intervals for inbred strain 13/n Guinea pigs (Cavia porcellus). J Am Assoc Lab Anim Sci 2019;58(3):293-303. DOI: https://doi.org/10.30802/AALAS-JAALAS-18-000118 [ Links ]
26. Kitagaki M, Yamaguchi M, Nakamura M, Sakurada K, Suwa T, Sasa H. Age-related changes in haematology and serum chemistry of Weiser-Maples guinea pigs (Cavia porcellus). Lab Anim 2005;39(3):321-30. DOI: https://doi.org/10.1258/0023677054307042 [ Links ]
27. Waner T, Avidar Y, Peh HC, Zass R, Bogin E. Hematology and clinical chemistry values of normal and euthymic hairless adult male Dunkin-Hartley guinea pigs (Cavia porcellus). Vet Clin Pathol 1996;25(2):61-4. DOI: https://doi.org/10.1111/j.1939-165x.1996.tb00971.x [ Links ]
28. Rodrigo-Condori NT, Flores-Merma H, Ramos-Zuñiga R, Cárdenas-Villanueva LA. Perfil bioquímico renal en cuyes (Cavia porcellus) alimentados con pisonay (Erythrina sp). Rev Inv Vet Perú 2020;31(4):e19249. DOI: http://dx.doi.org/10.15381/rivep.v31i4.19249 [ Links ]
29. Kumar R, Sharma R, Patil RD, Mal G, Kumar A, Patial V, et al. Sub-chronic toxicopathological study of lantadenes of Lantana camara weed in guinea pigs. BMC Vet Res 2018;14(1):129. DOI: https://doi.org/10.1186/s12917-018-1444-x [ Links ]
30. Holowaychuk MK. Renal failure in a guinea pig (Cavia porcellus) following ingestion of oxalate containing plants. Can Vet J 2006;47(8):787-9. [ Links ]
31. Vega Ugarte AO. Evaluación del perfil bioquímico sanguíneo de tres dietas en cuyes (Cavia porcellus) en etapa de crecimiento en una granja Comercial. Paucarpata-Arequipa 2016 ENT#091;tesis licenciaturaENT#093;. ENT#091;ArequipaENT#093;: Universidad Catolica de Santa Maria; 2016 ENT#091;citado 26 de mayo de 2023ENT#093;. Recuperado a partir de: https://repositorio.ucsm.edu.pe/handle/20.500.12920/6088 [ Links ]
32. Oze G, Nwanjo H, Onyeze G. Nephrotoxicity caused by the extract of Alstonia boonei (de Wild) stem bark in guinea pigs. Internet J Nutr Wellness 2006;3(2):1-10. [ Links ]
33. Braun JP, Lefebvre HP. Kidney function and damage. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical Biochemistry of Domestic Animals. Amsterdam: Elsevier B.V.; 2008. p. 485-528. DOI: https://doi.org/10.1016/B978-0-12-370491-7.00016-7 [ Links ]
34. Bauck L, Stefkovic G. Searching the records for clues about kidney disease in guinea pigs. Vet Med 1986;81:1127-30. [ Links ]
35. Fisher PG. Exotic mammal renal disease: causes and clinical presentation. Vet Clin North Am Exot Anim Pract 2006;9(1):33-67. DOI: https://doi.org/10.1016/j.cvex.2005.10.004 [ Links ]
36. López-Heydeck SM, López-Arriaga JA, Monte-negro-Morales LP, Cerecero-Aguirre P, Vázquez-de Anda GF. Análisis de laboratorio para el diag-nóstico temprano de insuficiencia renal crónica. Rev Mex Urol 2018;78(1):73-90. DOI: https://doi.org/10.24245/revmexurol.v78i1.1601 [ Links ]
37. Zapata Barrera JL, del Sol M, Vásquez B. Estereología renal en el cobayo (Cavia porcellus). Int J Morphol 2009;27(2):419-24. DOI: http://dx.doi.org/10.4067/S0717-95022009000200018 [ Links ]
38. Srinivas B, Somvanshi R, Biswas JC. Effect of feeding potato (Solanum tuberosum) plant on weight and haematology of guinea-pigs. Indian J Anim Nutr 1994;11(1):25-9. [ Links ]
39. Mustafa AF, Chavarr EC, Mantilla JG, Mantilla JO, Paredes MA. Effects of feeding flaxseed on performance, carcass trait, and meat fatty acid composition of guinea pigs (Cavia procellus) under northern Peruvian condition. Trop Anim Health Prod 2019;51(8):2611-7. DOI: https://doi.org/10.1007/s11250-019-01977-0 [ Links ]
40. Paredes M, Mantilla J, Bustamante I, Mantilla J, Cayotopa J, Hoban C, et al. Effects of five levels of dietary electrolyte balance on growth, carcass characteristics and blood serum metabolites of guinea pig (Cavia porcellus). Rev Investig Vet Peru 2021;32(2):e20018. DOI: http://dx.doi.org/10.15381/rivep.v32i2.20018 [ Links ]
41. Kerr MG. The nitrogenous substances. In: Kerr MG, editor. Veterinary laboratory medicine, Clinical biochemistry and haematology ENT#091;InternetENT#093;. Paris: Blackwell Science Ltd; 2002. p. 101-10. Retrieved from: https://www.cabidigitallibrary.org/doi/full/10.5555/20013180067 [ Links ]
42. Arsad SS, Esa NM, Hamzah H, Othman F. Evaluation of acute, subacute and subchronic oral toxicity of Rhaphidophora decursiva (Roxb.) Schott extract in male Sprague Dawley rats. J Med Plant Res 2013;7(41):3030-40. DOI: https://doi.org/10.5897/JMPR2013.2611 [ Links ]
43. Kifayatullah M, Mustafa MS, Sengupta P, Sarker MMR, Das A, Das, SK. Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.) Merr. in BALB/c mice. J Acute Dis 2015;4(4):309-15. DOI: https://doi.org/10.1016/j.joad.2015.06.010 [ Links ]
Acknowledgments To the Faculty of Veterinary Medicine and Animal Husbandry of the Universidad Nacional Micaela Bastidas de Apurimac, the Saavedra family and the company Del Corral E.I.R.L.
Ethical considerations We considered the vital space of the cages that allowed the welfare and tranquility of the guinea pigs, adequate diets were provided in accordance with nutritional requirements and fresh water was freely available, finally the guinea pigs were fed according to current regulations.
Authors' contribution to the articleJennefer Vega Cruz, experiment execution, drafting and preparation of the original draft. Ruth Ramos-Zuñiga, experiment execution and project administration. Ludwing Angel Cárdenas-Villanueva, supervision of results and discussion, information search, writing and final revision of the document for possible publication.
Editor's Note: Journal of the Selva Andina Animal Science (JSAAS). All statements expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, editors, and reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Received: June 01, 2023; Accepted: January 01, 2024











 texto en
texto en 
 uBio
uBio