Revista Boliviana de Química
versión On-line ISSN 0250-5460
Rev. Bol. Quim v.24 n.1 La Paz 2007
ORIGINAL ARTICLE
SEPARATION OF PHENOLIC COMPOUNDS FROM FOODS BY REVERSED-PHASE HIGH PERFORMANCE LIQUID CHROMATOGRAPHY
Instituto de Investigaciones Químicas, Universidad Mayor de San Andrés, La Paz , Bolivia, b Biomedical Nutrition, Pure and Applied Biochemistry, Lund University , Lund, Sweden, c Food Technology, Lund University, Lund, Sweden
Corresponding author: jaalvkir@gmail,com
J. Mauricio Peñarrieta abc , J. Antonio Alvarado a* , Björn ?kesson b and Björn Bergenståhl c
Departamento de Salud Pública, Facultad de Medicina. Universidad Nacional Autónoma de México. México, DF, México
RESUMEN
Como parte del estudio sobre el contenido de antioxidantes y compuestos fenólicos en alimentos bolivianos fue establecido un método rápido de cromatografía líquida de alta resolución usando una columna de fase reversa con detector por arreglo de diodos (HPLC-DAD). El método fue utilizado para la determinación de compuestos fenólicos en alimentos y fue lograda una buena resolución en diferentes compuestos estándares.
Palabras clave: plaguicidas; trabajadores agrícolas; género y salud; México
ABSTRACT
As a part of a study on the content of antioxidants and phenolic compounds in Bolivian foods, a rapid high performance liquid chromatographic method using a reversed-phase column and a diode array detector (HPLC-DAD) method was established. It was used for the determination of phenolic compounds in foods and a good resolution of different standard compounds was achieved
Key words: pesticides; agricultural workers; health and gender; Mexico
INTRODUCTION
performance liquid chromatography (RP-HPLC) (10). HPLC allows a high resolution and a rapid and reproducible determination, even of trace amounts of these compounds. Several methods have been developed using the RP-HPLC technique (11-13). However, it is necessary to design tailor-made methods depending on the nature of the samples. In the present work a rapid RP-HPLC method for the determination of different phenolic substances was established based on the method of Cristea et al. (14).
RESULTS AND DISCUSSION
Separation of phenolic standards by HPLC
Thirty-five standards of phenolic compounds were chromatographed by HPLC using a UV-vis diode array detector recording at 280, 360 and 530 nm. The method showed a good separation of most reference compounds. The retention time and UV maxima of the thirty-five standards are shown in Table 1. These data were used for the identification of the substances found in food samples.
Phenolic acids, catechins and other substances
Different amounts of phenolic acids, catechins, and other phenolic substances were injected separately and in mixtures into the HPLC. Standard curves were created and Figure 1 shows a calibration curve of quercetin obtained on two different days.

Figure 1. Calibration curve of quercetin standards
For this subgroup a good separation was obtained using a mixture of cinnamic acid, chlorogenic acid, ellagic acid, ferulic acid, benzoic acid, gallic acid, caffeic acid and vanillic acid as shown in Figure 2A.
Flavonoids
The HPLC method showed as well a good separation of a mixture of flavonoids. Six flavonoids were routinely used as reference compounds and a chromatogram using the measurement of the absorbance at 360 nm is shown in Figure 2B.
Anthocyanins
The maximum absorbance of anthocyanins is around 530 nm. This was used to measure anthocyanins which were injected into the HPLC either separately or in mixtures as shown in Figure 2C .
Concluding remarks
In conclusion the present work presents a convenient HPLC method for the separation of phenolic acids, flavonoids, anthocyanins and other phenolic substances from foods. Using methanol and acetic acid (1%) in water as solvents, good separations were obtained rapidly. The method showed a good reproducibility for the separation of different standard compounds. Absorbance data from the diode array detector were obtained at different wavelengths for the reference substances. The absorbance spectra and the retention times were used for the identification of the substances. In future studies additional confirmation of the identity of different compounds in foods isnecessary using mass spectrometry and other techniques. Quantification of the substances in food was performed using external standard curves . The present method can be considered as a valuable alternative and complementary method to different spectrophotometric methods (15) for the study of the content of different phenolic substances in foods and other natural sources.
EXPERIMENTAL
Chemicals
G allic acid was purchased from Merck (Darmstadt, Germany), baicalein (98%), 3,4-dihydroxybenzaldehyde (99%benzoic acid (99%), catechin (99% ), caffeic acid (99%), catechin gallate (99%), catechol (99%), 4-methylcatechol (99%), 5-methylcatechol (99%), tert.butylcatechol (99%), chlorogenic acid (99%), ellagic acid (99)%, epicatechin (99%), guiachol (99%), hydroquinone (99%), kaempferol (99%), morin (99%), myricetin (99%), naringenin (99%), pyrogallol (99%), quercetin (99%), resorcinol (99%), 4-methylresorcinol (99%), 5-methylresorcinol (99%), rutin (99%), vanillic acid (99%), and vanillin (99%) were obtained from Sigma-Aldrich (St. Louis, USA), acetic acid (glacial p.a.), ferulic acid, d elphininCl, cyanidinCl, m alvidinCl, pelargonidinCl and p etunidinCl were obtained from Extrasynthèse (Genay, France), and methanol HPLC grade from Laboratory supplies (Poole, U.K.).

Figure 2, Chromatograms of different phenolic substances obtained by the HPLC method.
Panel A (upper). The detector was set at 280 nm mainly for phenolic acids. A: gallic acid; B: resorcinol; C: vanillic acid; D: 4-methylresorcinol; E: ellagic acid; F: benzoic acid; G: cinnamic acid. Panel B (middle). The detector was set at 360 nm for flavonoids. A: rutin; B: myricetin; C: morin; D: quercetin; E: kaempferol; F: baicalein (used as an internal standard). Panel C (lower). The detector was set at 530 nm for anthocyanins. A: delphinidin; B: cyanidin; C: petunidin; D: pelargonidin; E: malvidin
Sample Preparation
The standards were dissolved in ethanol HPLC grade at different concentration and filtered by 0.22 µm sterile Millex filters purchased from Millipore ( Bedford , U.K. ) before the injection.
High performance liquid chromatography
Before the HPLC analysis, the water-insoluble fractions were evaporated in a nitrogen stream and reconstituted in methanol and after that both the water-soluble and water-insoluble fractions were refluxed in 1.5 M HCl in methanol for 90 min for the hydrolysis of glycosides. Baicalein was added as an internal standard before the hydrolysis. Phenolic compounds were separated using a Shimadzu liquid chromatograph system (LC 10ADVP), comprising a vacuum degasser (DGU 14-A), a solvent delivery module (FCV-10ALVP), an auto-injector (SIL-10ADVP), a column oven (CTO-10ASVP) and a diode-array detector (SPD-M10AVP). The column was a 3.5 mm Kromasil reversed-phase column 150 mm x 4 mm protected by a Kromasil C 18 10 mm pre-column (Scantec Lab, Sävedalen, Sweden). The flow rate was 0.8 ml/min and the injection volume was 20 µ l. The mobile phase was a binary solvent system consisting of (A) methanol and (B) 1% acetic acid/water and the gradient used was 0 min 40% B, 5 min 65% B, 10 min 90% B, 15 min 40% B until 17 min as modified from (14). The UV absorbance at 280, 360 and 530 nm was recorded in the eluate. The compounds were identified by comparing with standards of each identified compound using the retention time, the absorbance spectrum profile and also by running the samples after the addition of pure standards. The chromatographic and spectral features of the standards are shown in Table 1. The concentrations were calculated from the peak heights of the internal standard and each compound in the samples and in reference solutions.
ACKNOWLEDGEMENTS
The study was supported by the Swedish International Development Agency (SIDA/SAREC) in a collaborative project between Universidad Mayor de San Andrés ( Bolivia ) and Lund University ( Sweden ). Additional support was provided by Lund University Hospital , the Påhlsson Foundation and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS).
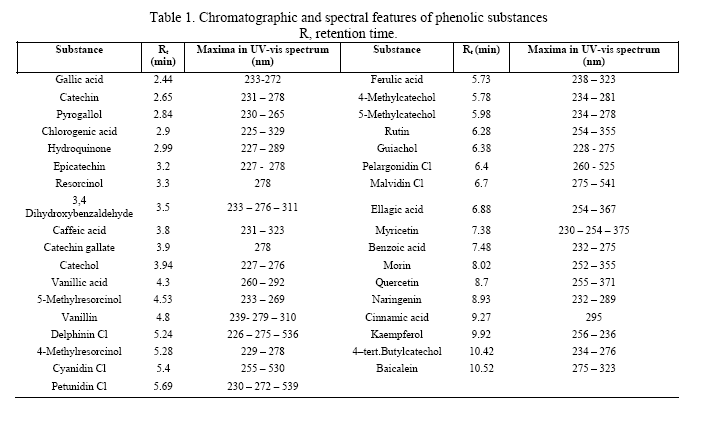
Table 1 . Chromatographic and spectral features of phenolic substances
R, retention time.
Referencias
1. HALVORSEN, B.L., HOLTE, K., MYHRSTAD, M.C.W., BARIKMO, I., HVATTUM, E., FAGERTUN REMBERG, S., WOLD, A.-B., HAFFNER, K., BAUGERØD, H., FROST ANDERSEN, L., MOSKAUG, J.Ø., JACOBS, D.R., JR., & BLOMHOFF, R. A systematic screening of total antioxidants in dietary plants. Journal of Nutrition, 2002, 132, 461-471.
2. NILSSON, J., PILLAI, D., ÖNNING, G., PERSSON, C., NILSSON, Å., & ÅKESSON, B. Comparison of the ABTS and FRAP methods to assess the total antioxidant capacity in extracts of fruit and vegetables. Molecular Nutrition and Food Research, 2005, 49, 239-246.
3. PEÑARRIETA, M., ALVARADO, J.A., AKESSON, B., & BERGENSTAHL, B. Antioxidant capacity in Andean food species from Bolivia . Revista Boliviana de.Química, 2005, 22, 89-93. [ Links ]
4. STEINMETZ, K.A., & POTTER, J.D. Vegetables, fruit and cancer prevention: A review. Journal of American Dietetic Association, 1996, 96, 1027-1039. [ Links ]
5. HERTOG M.G.L., HOLLMAN P.C.H., & VENEMA, D.P. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. Journal of Agricultural and Food Chemistry, 1992, 40, 1591-1598. [ Links ]
6. BENZIE, I.F.F., & STRAIN, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power The FRAP assay. Analytical Biochemistry, 1996, 239, 70–76. [ Links ]
7. RE, R., PELLEGRINI, N., PROTEGGENTE, A., PANNALA, A., YANG, M., & RICE- EVANS, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radicals in Biology and Medicine, 1999, 26, 1231–1237. [ Links ]
8. SINGLETON, V.L., & ROSSI, J.A. Jr., Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. American Journal of Enology and Viticulture, 1965, 16, 144-158. [ Links ]
. ZHISHEN, J., MENGCHENG, T., & JIANMING, W. Determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 1999, 64, 555-559.
10. MERKEN, H. M., & BEECHER, G. R. Measurement of food flavonoids by high-performance liquid chromatography: A review. Journal of Agricultural and Food Chemistry, 2000 , 48 , 577-599. [ Links ]
11. MÄÄTTÄ, K.R., KAMAL-ELDIN, A., & TÖRRÖNEN A.R. High performance liquid chromatography (HPLC) analysis of phenolics compounds in berries with diode array and electrospray ionization mass spectrometric (MS) detection: Ribes Species. Journal of Agricultural and Food Chemistry, 2003, 51, 6736-6744. [ Links ]
12. SUÁREZ, B., PALACIOS, N., FRAGA, N., & RODRÍGUEZ, R. Liquid chromatographic method for quantifying polyphenols in ciders by direct injection. Journal of Chromatography A, 2005, 1066, 105–110. [ Links ]
13. LU, K.L., KU, Y.R., WEN, K.C., HO, L.K., & CHANG, Y.S. Analysis of flavonoids and coumarins in ixeris laevigata var. oldhami by HPLC. Journal of Liquid Chromatography and related technologies, 2000, 23, 2573–2583. [ Links ]
14. CRISTEA, D., BEREAU, I. , & VILAREM, G. Identification and quantitative HPLC analysis of the main flavonoids present in weld (Reseda luteola L.). Dyes and Pigments, 2003, 57, 267-272. [ Links ]
15. PEÑARRIETA J.M., ALVARADO J.A., BERGENSTÅHL, B., & ÅKESSON, B. Spectrophotometric methods for the measurement of total phenolic compounds and total flavonoids in foods (to be published) [ Links ]












 uBio
uBio