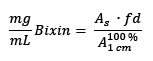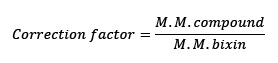INTRODUCTION
Carotenoids are lipophilic pigments that are biosynthesized by photosynthetic organisms, mainly by plants 1. The carotenoid’s biological properties rely on their molecular structure 2. The conjugated polyene system of the carotenoids allows them to stabilize radical molecules such as reactive oxygen species (ROS) 2), (3.
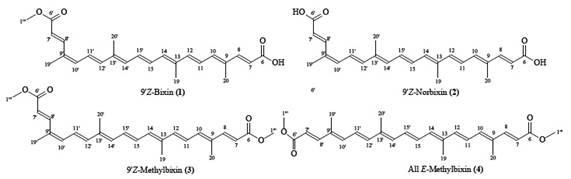
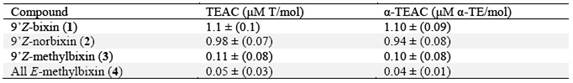
Commercial annatto preparations are available for imparting yellow, orange or red coloration to products such as butter, cheese, sausages and dressings 4. In the food industries, annatto is known as E-160b or Natural Orange 4),(6. It could be of commercial interest that a dye provides more than just color, meaning that it could contribute to a healthy diet. 9´Z-bixin (1), a subclass of carotenoids, named apocarotenoids, is the major pigment present in the annatto (Bixa orellana L.) seeds 7. 9´Z-bixin (1) represents approximately 80% of the carotenoids 4 and is the first naturally occurring (Z)-carotenoid 8. Other minor carotenoids present in the annatto seeds are 9´Z-norbixin (2) and 9´Z-methylbixin (3)4. See Fig.1
Studies conducted on the effects of bixin and norbixin on rats, in which diabetes was induced with streptozotocin, showed that bixin helps to lower blood glucose levels and prevents oxidative stress. The water-soluble analog of bixin, norbixin, turned out to be inactive against diabetes 9 Other study, on liver cancer cell lines, have provided evidence that bixin can produce apoptosis, in addition to sensitizing cancerous cells to the action of dacarbazine, an antineoplastic chemotherapy drug used in the treatment of various types of cancer 10.
Other studies indicate that the extract of annatto seeds has antimicrobial activity on some strains of bacteria such as Bacillus subtilis, Streptococcus faecalis, Staphylococcus aureus 11 and Proteus mirabilis 12. In addition, studies of toxicity in rats have shown that bixin has little or no toxicity in rats that were administered 1000 mg per kilogram of body weight 13.
Reactive oxygen species (ROS) have the potential to give rise to cancer, cardiovascular diseases, fibrotic diseases, chronic inflammation, and autoimmune diseases 14. This has led to an increase in the interest of the antioxidant capacity of biomolecular compounds present in foods 5.
The α-tocopherol/Trolox equivalent antioxidant capacity (α-TEAC/TEAC) assay is a decolorization assay that uses the radical ABTS•+ to measure the antioxidant capacity. The radical is generated by the oxidation of ABTS with potassium persulfate 15 or manganese dioxide 16 and is reduced in the presence of antioxidants 16),(18. This assay is fundamental in the electron transfer reaction and allows for the antioxidant capacity to be measured 16),(18.
The purpose of this study was to measure the antioxidant capacity of different bixin derivatives by the ATBS assay and relate it to its chemical structure. This, to show how esterification reactions affect the antioxidant activity of bixin derivatives compared to 9´Z-bixin.
RESULTS AND DISCUSSION
Extraction and isolation of bixin from annatto seeds
Annatto (Bixa orellana L.) seeds were extracted with acetone, yielding a mixture containing 1.42% total carotenoids of seeds, and 66.81% natural bixin (by HPLC Area, Fig.1 ) of the total carotenoids extract. The quantifications were performed according to FAO/WHO Joint Expert Committee on Food Additives 5 detailed in the experimental section (Sample quantification) where the percentage was determined by HPLC-SPD at 450 nm using the peak area in the chromatogram. Data from literature 4 indicates that approximately 80% of the naturally occurring carotenoids present in the annatto seeds is bixin (1), (Fig.1) In this sense, the lower percentage obtained could be due to the variety of annatto acquired, or the post-harvest seed treatment 19),(20. The isolation of natural 9’Z-bixin (1), (Fig.1) from annatto seeds was achieved with a peak in the HPLC-SPD chromatogram at 450 nm (100% of the total area), a fact indicating a high degree of purity.
Hemisynthesis of bixin derivatives
Hemisynthetic 9´Z-norbixin (2) (Fig.1) was obtained by the saponification of annatto seeds extract with 5% potassium hydroxide in methanol 21, with a yield of 85% of the total carotenoids extract, determined by HPLC-SPD area at 450 nm.
HPLC run with the products of saponification of the crude extract of total carotenoids of the seeds for the obtaining of (2) (Fig.1) also allowed the obtaining of 9’Z-methylbixin (3) (Fig.1) with a high degree of purity, HPLC-SPD chromatogram at 450 nm (91 % of the total area).
Hemisynthetic 9´Z-methylbixin (3) was obtained from natural 9’Z-bixin (1) through Steglich esterification of the later 22, with the use of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and dimethylaminopyridine (DMAP). The yield was 74% of (1). This methodology is new for the obtention of 9´Z-methylbixin. Previously, hemisynthetic methylbixin was obtained via reaction of naturally occurring 9´Z-bixin with diazomethane or dimethylsulfate 23. Both methodologies required very expensive and specialized equipment 24, and the use of these reagents can cause genetic damage to the users that perform the reaction 25. The use of EDC/DMAP allowed for hemisynthetic 9´Z-methylbixin (3) (Fig.1) to be obtained in a simple, economic way and with good yields. The isolation of (3) occurred with a high degree of purity according to HPLC-SPD chromatogram at 450 nm with 100 % of the total area.
Hemisynthetic E-methylbixin (4) was obtained by esterification in acid conditions (HCl + CH3OH), of compound (1). The compound was obtained with a yield of 34% of (1) determined by HPLC-SPD area at 450 nm. The low yield may be due to the high concentrations of acid used or the prolonged reaction time. It is reported in the literature that high concentrations of acid produce carotenoid bleaching, meaning that the carotenoid is degraded in lower molecular weight components that do not absorb in the visible region 26. The isolation of (4) presented a peak in the HPLC-SPD chromatogram at 450 nm of 97 % of the total area, which indicated a high degree of purity.
The 1H- and 13C-NMR, UV/VIS, MS and MS2 data of bixin (1) and bixin derivatives (2), (3) and (4) are in full agreement with the data reported in the literature 7), (23), (27), (28.
Evaluation of the antioxidant capacity with the radical ABTS•+
Table 1 shows the TEAC and α-TEAC results. Compounds (1) and (2) showed the highest TEAC and α-TEAC values. They did not present significant differences between values obtained in their respective assays. The same can be said for compounds (2), (3) and (4). This reveals that there are no significant differences between the antioxidant capacity of α-tocopherol and Trolox 29.
The results showed that the esterification [nucleophilic substitution that gave rise to a methoxy group out of a hydroxy group that was replaced in the carboxylic acid residue (C-6) of carotenoid (1), to afford (3) or (4), Fig.1] decreased the TEAC and α-TEAC values. Evidence indicates that the aforementioned substitution almost completely decreases antioxidant capacity. Moreover, there are no differences in the antioxidant activity between compounds (1) and (2) revealed by both assays (TEAC and α-TEAC).
On the other hand, no differences between compounds (3) and (4) were observed regarding their antioxidant capacity, according to the currently employed antioxidant assays. In spite of the opposed stereochemistry at the C9’=C10’ bond, Z [(3)] versus E [(4)], the difference in the ability to quench ABTS radicals between these compounds, was irrelevant.
In Fig. 1, compound (1) with one terminal acid function (C-7,) and (2) with two (C-7 and C-7’), showed more antioxidant capacity, than (3) and (4), with ester functions at such positions . The derivatization seems to be the cause for the difference in the ABTS indexes (Table 1).
Is very important to recognize that (1) and (2) are the major compounds in the commercial annatto extract [30] and have a high antioxidant capacity compared with the bixin derivatives.
In the scavenging of the radical ABTS•+ in the presence of such hydrogen-donating antioxidants 15, it seems that the acid proton of the acid function, could be responsible for the antioxidant capacity. It is possible that esterification (no acid proton) limits bixin-derivatives’ antioxidant capacity. The terminal acid function shows the possibility for decarboxylation (-CO2) with formation of ·H, this is not possible for derivatives.
EXPERIMENTAL
General Experimental Procedures
Bixin extract and fractions were isolated using a semipreparative HPLC system (HP 1050 Series, Hewlett-Packard/Agilent Technologies Co., Palo Alto, CA, USA), equipped with a ProntoSil C30 of 200 x 3,0 mm column (Bischoff, Leonberg, Germany). A separation was performed by gradient elution (2.5 ml/min) started with 100% of solvent A until reached 100% of solvent B in 50 min. Solvent B was a mixture of 90% methyl t-butyl ether: 6% methanol: 4% water. Solvent A was a mixture of 15% methyl t-butyl ether: 81% methanol: 4% water. Data analysis was performed using the OpenLab CDS data system.
The UV-vis spectra were recorded with a Genesys 10S UV-Vis spectrometer (Thermo Scientific, Waltham, MA, USA). The MS and MS2 experiments were performed using a Waters UPLC ACQUITY class H coupled with XEVO TQD spectrometer (Waters Corporations, Milford, MA, USA). The samples were dissolved in MeOH (HPLC-grade) and measured in positive APCI mode. The samples were filtered in a 0.22 µm filters and analyzed with the following conditions: a cone voltage of 30 V, corona voltage of 5 V, probe temperature of 550 °C, carrier gas desolvation flow of 500 L/h, cone flow of 50 L/h and collision energy of 3 V. For the MS2 spectra, the collision energy was increased between 7 and 20 V. The 1H NMR (600 MHz), 13CNMR (151 MHz), and 2D NMR experiments 1H-1H COSY, HMBC and HSQC were run in a Bruker Avance 600 MHz NMR spectrometer using Bruker microprograms. The samples were dissolved in DMSO-d6 for (1) and (2) or CDCl3 for (3) and (4). Chemical shifts were measured from internal TMS or residual solvent signals. UV, MS and NMR spectra available as supplementary material.
Plant material
Annatto (Bixa orellana L.) seeds were bought in a local market in Panama City (MERCA PANAMA, Panama).
Bixin Isolation
200 g of seeds were extracted with acetone (2000 mL). The acetone extract was dried on a rotary evaporator. 1 g of dried annatto seed extract was chromatographed on a 60 g silica gel column with a gradient of diethyl ether in hexane. The isolated bixin fraction was purified by semipreparative HPLC-SPD on a ProntoSil C30 250 x 10,0 mm (Bischoff, Leonberg, Germany) column.
Sample Quantification
The bixin and bixin derivatives content was determined by spectrophotometric methodology using to specific absorption coefficient and according to the FAO/WHO Joint Expert Committee on Food Additives 5. The sample’s absorbance was measured in a 1 cm quartz cuvette at 487 nm using a UV-visible spectrophotometer and acetone as a blank. The contents of bixin in the samples were calculated with the following equation 5:
in which:
As= the absorbance of the samples;
Fd = the dilution factor;
A100% 1 cm3090 (1g/100 mL)-1∗1 cm-1 for bixin in acetone (specific absorption coefficient).
The concentration was expressed as total carotenoids in bixin form, and the purity was determined by HPLC-SPD at 450 nm using the peak area in the chromatogram.
To correct the quantification of compounds (2), (3) and (4) that were structurally different from bixin, but they have very similar UV-Vis spectra, a correction factor calculated as follows was used:
in which:
M.M. = The molecular mass of the compound;
M.M. = The molecular mass of bixin (394.5 g/mol).
This methodology was used in the quantification of all the samples in this work.
9´ Z-Norbixin (2)
Bixin (0.13 mmol, 50 mg) in 50 mL of dry diethyl ether, was dissolved in a 5% KOH methanolic solution. The sample was stirred in a Schlenk flask for five hours at 30 °C. At the beginning, 20 mL of chlorohydric acid (37 % w/w) was added slowly, and the diluted sample was transferred to a separation funnel and washed with diethyl ether (four times with 250 ml each), and 1 L of deionized water until it reached a neutral pH. The organic phase was evaporated in vacuo to obtain the products. The residue was rediluted in a mixture of 90 methyl t-butyl ether: 6 methanol: 4 water, for isolation. The desired compound was isolated by semipreparative HPLC-SPD, with a ProntoSil C30 250 x 10,0 mm column (Bischoff, Leonberg, Germany). Chromatographic separation was performed by using an isocratic mixture (90 methyl t-butyl ether: 6 methanol: 4 water) and flow of 2 mL/min.
9' Z-Methylbixin (3)
A total of 98 mg (0.51 mmol) of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) (2 equivalent), 0.3 mL of pyridine (1.19 mmol) (4.7 equivalent) and 8 mg (0.065 mmol) of dimethylaminopyridine (DMAP) were added to bixin (0.25 mmol, 100 mg) (1 equivalent) in dry dichloromethane (DCM). The sample was stirred for 24 hours at 30 °C. The sample was washed with HCl 2% (three times with 20 ml each), 5% NaHCO3 (Three times with 20 ml each) and 50 mL of deionized water. The combined organic phase was evaporated in vacuo to obtain the products. The residue was adsorbed in three grams of Silica gel 60 and eluted in a column packed with Silica gel 60, with a gradual increment of diethyl ether in a hexane solution. The fraction with the highest content of the product was isolated by semipreparative HPLC-SPD with a ProntoSil C30 250 x 10,0 mm column (Bischoff, Leonberg, Germany). Chromatographic separation was performed using an isocratic mixture (90 methyl t-butyl ether: 6 methanol: 4 water) and flow of 2.5 mL/min.
All E-Methylbixin (4)
A total of 30 mL of a solution of acidified methanol (5 M) was added with bixin (0.78 mmol, 300 mg) in dry methanol, which was prepared by bubbling dry hydrogen chloride in methanol. The sample was stirred for 24 hours at 60 °C. A total of 10 g of sodium bicarbonate solid was slowly added to the mixture, evaporated in vacuo, dissolved with 200 mL of DCM, and washed with 400 mL of deionized water. The organic phase was evaporated in vacuo to obtain the products. The residue was adsorbed in 3 g of silica gel 60 and eluted in a column packed with silica gel 60 with a gradient of diethyl ether in a hexane solution. The fraction with the major content of the product was isolated by semipreparative HPLC-SPD with a ProntoSil C30 250 x 10,0 mm column (Bischoff, Leonberg, Germany). Chromatographic separation was performed by using an isocratic mixture (90 methyl t-butyl ether: 6 methanol: 4 water) and flow of 2 mL/min.
Evaluation of the antioxidant capacity with the radical ABTS•+
The following experimental procedures were applied to evaluate the antioxidant capacity of 9´Z-bixin and bixin derivatives and, when possible, structure-activity relationships. Before analysis, Stock solutions of bixin and bixin derivatives (50 µmol/L) were prepared in diethyl ether and 2 mL were transferred to an amber vial. All were evaporated under a stream of nitrogen at 30 ± 1 °C. The samples were stored dry at -20° C. Exact concentrations of the stock solutions were determined spectrophotometrically using the methodology previously described. For the analysis, the samples were redissolved in 1 mL of methanol (100 μM), successive dilutions were prepared at 100, 70, 30 and 10 μM. All the carotenoid samples were analyzed in triplicate at four different concentrations (100, 70, 30 and 10 μM).
The Trolox equivalent antioxidant capacity (TEAC) and α-tocopherol equivalent antioxidant capacity (α-TEAC) procedure used an automated plate reader (KC4, Bio Tek, USA) with 96-well plates. Procedures for measuring the TEAC and α-TEAC values were carried out using Mϋller et al.’s (2010) method [29] with a few modifications. The radical solution of ABTS•+ was prepared by passing an ABTS solution in 5 mM phosphate-buffered saline (PBS) solution, with pH 7.4, through a filter paper coated with manganese dioxide. This was followed by filtration with a syringe filter with a pore size of 0.22 μm. Before analysis, the stock solution of ABTS was diluted with PBS to obtain an absorbance of 0.75 ± 0.05 at 734 nm and stabilized for 2 hours. In the assay, 40 μL of carotenoid solution, Trolox (ca. 4.7-117.5 μM), α-tocopherol (ca. 4.4-111.6 μM) or blank (methanol) and 240 μL of ABTS were stirred for 30 s. Thereafter, the absorbance was determined at 734 nm for 20 min. For the TEAC, Trolox was used as a standard, and α-tocopherol was used as a standard for α-TEAC.
Statistics
Each sample analysis was performed in triplicate. All the presented results are the means (±SD) of at least three independent experiments. Statistical analysis (ANOVA with a statistical significance level set at p < 0.05, with a post hoc Student-Newman-Keuls (S-N-K) procedure) was carried out with SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
CONCLUSION
Compounds (1) and (2) with a free carboxyl group showed a high antioxidant activity, similar to Trolox and α-tocopherol. The free carboxyl group is of importance for the antioxidant capacity of bixin. The bixin derivatives (3) and (4) showed a low antioxidant capacity, which could be due to the loss of the free carboxyl group.
SUPPLEMENTARY MATERIAL
The following supporting information can be downloaded at: http://www.bolivianchemistryjournal.org/ (Vol. 39, No. 3, 2022, August 30). Figure S1: NMR spectra of compound (1), a) 1H, b) 13C, c) COSY, d) HSQC. Figure S2: NMR spectra of compound (2), a) 1H, b) 13C, c) COSY, d) HSQC. Figure S3: NMR spectra of compound (3), a) 1H, b) 13C, c) COSY, d) HSQC. Figure S4: NMR spectra of compound (4), a) 1H, b) 13C, c) COSY, d) HSQC. Figure S5: Mass spectrum of a) (1), b) (2), c) (3) and (4). Figure S6: UV-vis spectrum of a) (1), b) (2), c) (3) and d) (4).












 uBio
uBio